Carbon Ring Structure to be Essentional for Proton and Lithium Permeation Through Graphene
May 19, 2020 - In a new study, researchers from the Universities of Manchester, Antwerp, Singapore, Jena, Ulm, Tokyo, and Beijing further explore graphene membranes as protective barriers in various Li-ion and hydrogen technologies.
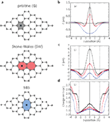
According to a comprehensive new study for both proton and Li-ion permeation through carbon ring structures (Figure 1), researchers found that the energy barrier for the transport across a graphene membrane decreased as the number of atoms within the rings increased (Figure 2), consistent with previous theoretical results.[1,2]
The results showed that proton and Li-ion transport through disordered graphene depends on the relative contributions of eight-atom rings in the samples.[3,4] The DFT calculations along with the experimental observations also explain why protons permeate disordered graphene about 10 times faster than Li-ions (Figure 3): Eight-atom rings provide an energy barrier for incoming Li-ions that is about twice as high as that for protons.
In the new study, the researchers also show that their interpretation is consistent with the lack of proton/Li-ion selectivity in carbon nanomembrane (CNM), which do not have eight-atom ring structures, but rather relatively large (~ 0.7 nm) nanopores. Indeed, for CNM, the H as well as the Li ions transport has been measured to be dependent on the CNM thickness, indicating that for CNM it is the mass transport that is important and not the energy barrier transport as in the case of graphene materials. The conclusion about mass transport through CNMs is also consistent with their microscopic structure, being effectively a dense network of subnanometer pores,[5] such that ions diffuse along tortuous trajectories, as is typical of porous media.[6]
For their study, the scientists used on the one hand side a patchwork of nanometer-sized graphene crystallites (3−4 nm). They called this material nanocrystalline defective graphene (DG, Figure 1a, b).[4] The grain boundaries of the material consist of rings with five, six, seven and eight atoms. Due to the small domain size, the material contains a high density of non-hexagonal structures.
On the other hand, the scientists used monolayer amorphous carbon (MAC),[3] which they synthesized by laser-assisted chemical vapor deposition. It is made up of an amorphous array of one atom thick rings with five, six, seven, and eight carbon atoms (Figure 1c, d) with no visible graphite crystallites.
The two DG films were compared with two other reference materials: Firstly with the nanometer-thick carbon film, the CNM. This material was synthesized by self-assembly from aromatic precursors that were cross-linked by electron irradiation, resulting in short-order molecular nanosheets.[5,7] CNM consist of a dense (~ 1014 cm-2) network of subnanometer pores (~ 0.7 nm in diameter) permeating the CNMs.[5] The CNMs used had thicknesses of ~ 0.9 and 1.2 nm. The second reference material were pristine graphene crystals obtained by mechanical exfoliation. This approach enabled - for the first time - to compare the permeability of 2D carbon materials over the entire range from crystalline to disordered structures.
To be able to compare the results with the number of ring structures, the team used HRTEM and STEM characterization. For the STEM characterization of amorphous monolayer carbon, the scientists at the AIST in Tokyo/Japan used a JEOL 2100F with a Delta probe aberration corrector. The acceleration voltage used was 60 kV, the convergent illumination angle was 35 mrad and the detector angle was in the range from 45 to 200 mrad. For STEM images, the samples were heated to 700 °C using the heater holder in the microscope. In the SALVE Microscopy Centre at Ulm University/Germany, measurements were carried out using 80 kV Cs-corrected HRTEM at the FEI-TITAN.
The development of defects in graphene and other 2D materials has far-reaching consequences for the material properties in energy conversion and energy storage technologies. The research was funded by the European Research Council, the Graphene Flagship, and the German Research Foundation.
Comparing the performance of different membranes Despite being a one-atom-thick material, no more than a few gas atoms per hour can permeate through micrometer-sized defect-free graphene membranes, as proven experimentally.[8,9] The dense electron clouds of graphene’s crystal lattice forbid permeation under ambient conditions imposing over an energy barrier of several electron-volts.[1,10] Now, the team wanted to prove if graphene's lattice can be modifieed by introducing seven- or eight-atom rings that should greatly reduce the energy barriers faced by protons[1] or small ions (eg, Li +) to permeate without losing graphene's impermeability with respect to atoms and molecules.[10]
Until know, enough graphene samples with high density of atomic-scale defects were generally not available. To create defects, graphene crystals were previously perforated using ion irradiation or chemical and plasma etching.[11,12] This approach results in a local loss of carbon atoms that typically form nanometer-sized pores[11] rather than atomic-scale defects. These nanopores are permeable to gases, ions, and even macromolecules (e.g., DNA)[11] The scientists reported proton and lithium-ion transport through disordered graphene grown using the methods reported previously.[3,4]
Moreover they identified the proton areal conductivity of disordered graphene at ∼60 ° C: It should exceed the industry benchmark set by Nafion 117 (∼5 S cm-2).[13] Defect-free graphene reaches this level only at ∼200 °C. The latter temperature is most desirable for fuel-cell operation.[13,15] By extrapolation of the measured σ(T), the DG membranes can reach ∼100 S cm-2 for this T range, well above the industry targets.[14]
Disordered graphene (DG) may be mass produced [3,4] and may act as a material to host highly reactive Li-metal[16] and Li-Si particles[17] as anodes and Li-sulfur as cathodes[18] in batteries. Thus, the lithium permeability of DG deserves special attention. Graphene is explored here to protect chemical reactions with electrolytes, prohibit Li dendritic growth, and provide mechanical stability.
Ute Kaiser (SALVE Microscopy Center) says: “I've been working with two-dimensional amorphous structures since 2009 and we see now that disordered 2D materials are a very promising candidate for filtration of protons or ions."[19]
Looking forward, the key properties needed for the filtration applications are high Li-ion conductivity combined with impermeability to reactive species. Because defect-free graphene is impermeable to Li ions, defects are essential, in particular those, permeable only to Li ions (like eight-atom rings) offer considerable advantages.
Resource: Griffin, E., Mogg, L., Hao, G. P., Kalon, G., Bacaksiz, C., Lopez-Polin, G., Zhou, T.Y., Guarochico, V., Cai, J., Neumann, C., Winter, A., Mohn, M., Lee, J. H., Lin, J., Kaiser, U., Grigorieva, I. V., Suenaga, K., Özyilmaz, B., Cheng, H. M., Ren, W., Turchanin, A., Peeters, F. M., Geim, A, K, & Lozada-Hidalgo, M. (2020) Proton and Li-Ion Permeation through Graphene with Eight-Atom-Ring Defects. ACS Nano, 14, 7280, doi: 10.1021/acsnano.0c02496.
-
Miao, M.; Nardelli, M. B.; Wang, Q.; Liu, Y., First principles study of the permeability of graphene to hydrogen atoms., Phys. Chem. Chem. Phys. 2013, 15, 16132−16137.
-
Xin, Y.; Huang, A.; Hu, Q.; Shi, H.; Wang, M.; Xiao, Z.; Zheng, X.; Di, Z.; Chu, P. K., Barrier reduction of lithium ion tunneling through graphenee with hybrid defects: first-principles calculations., Adv. Theory Simul. 2018, 1, 1700009.
-
Toh, C.-T.; Zhang, H.; Lin, J.; Mayorov, A. S.; Wang, Y.-P.; Orofeo, C. M.; Ferry, D. B.; Andersen, H.; Kakenov, N.; Guo, Z.; Abidi, I. H.; Sims, H.; Suenaga, K.; Pantelides, S. T.; Özyilmaz, B., Synthesis and properties of free-standing monolayer amorphous carbon., Nature 2020, 577, 199−203.
-
Zhao, T.; Xu, C.; Ma, W.; Liu, Z.; Zhou, T.; Liu, Z.; Feng, S.; Zhu, M.; Kang, N.; Sun, D.-M.; Cheng, H.-M.; Ren, W., Ultrafast growth of nanocrystalline graphene films by quenching and grain-size-dependent strength and bandgap opening., Nat. Commun. 2019, 10, 4854.
-
Yang, Y.; Dementyev, P.; Biere, N.; Emmrich, D.; Stohmann, P.; Korzetz, R.; Zhang, X.; Beyer, A.; Koch, S.; Anselmetti, D.; Gölzhauser, A., Rapid water permeation through carbon nanomembranes with sub-nanometer channels., ACS Nano 2018, 12, 4695−4701.
-
Wiedenmann, D.; Keller, L.; Holzer, L.; Stojadinovic, J.; Munch, B.; Suarez, L.; Fumey, B.; Hagendorfer, H.; Bronnimann, R.; Modregger, P.; Gorbar, M.; Vogt, U. F.; Zuttel, A.; Mantia, F. L.; Wepf, R.; Grobety, B., Three-dimensional porous structure and ion conductivity of porous ceramic diaphragms., AIChE J. 2013, 59, 1446−1457.
-
Turchanin, A.; Gölzhauser, A., Carbon Nanomembranes., Adv. Mater. 2016, 28, 6075−6103.
-
Sun, P. Z.; Yang, Q.; Kuang, W. J.; Stebunov, Y. V.; Xiong, W. Q.; Yu, J.; Nair, R. R.; Katsnelson, M. I.; Yuan, S. J.; Grigorieva, I. V.; Lozada-Hidalgo, M.; Wang, F. C.; Geim, A. K., Limits on gas impermeability of graphene., Nature 2020, 579, 229−232.
-
Mogg, L.; Zhang, S.; Hao, G.-P.; Gopinadhan, K.; Barry, D.; Liu, B. L.; Cheng, H. M.; Geim, A. K.; Lozada-Hidalgo, M., Perfect proton selectivity in ion transport through two-dimensional crystals., Nat. Commun. 2019, 10, 4243.
-
Leenaerts, O.; Partoens, B.; Peeters, F. M., Graphene: A perfect nanoballoon., Appl. Phys. Lett. 2008, 93, 193107.
-
Wang, L.; Boutilier, M. S. H.; Kidambi, P. R.; Jang, D.; Hadjiconstantinou, N. G.; Karnik, R., Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes., Nat. Nanotechnol. 2017, 12, 509−522.
-
Kotakoski, J.; Krasheninnikov, A. V.; Kaiser, U.; Meyer, J. C., From point defects in graphene to two dimensional amorphous carbon., Phys. Rev. Lett. 2011, 106, 105505.
-
Casciola, M.; Alberti, G.; Sganappa, M.; Narducci, R. , On the decay of Nafion proton conductivity at high temperature and relative humidity., J. Power Sources 2006, 162, 141−145.
-
U.S Department of Energy., Multi-year research, development and demonstration plan., https://www.energy.gov/sites/prod/files/2014/12/f19/fcto_myrdd_full_document.pdf (accessed 2018-06-01).
-
Bose, S.; Kuila, T.; Nguyen, T. X. H.; Kim, N. H.; Lau, K.-T.; Lee, J. H., Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges., Prog. Polym. Sci. 2011, 36, 813−843.
-
Zhao, J.; Zhou, G.; Yan, K.; Xie, J.; Li, Y.; Liao, L.; Jin, Y.; Liu, K.; Hsu, P.-C.; Wang, J.; Cheng, H.-M.; Cui, Y., Air-Stable and freestanding lithium alloy/graphene foil as an alternative to lithium metal anodes., Nat. Nanotechnol. 2017, 12, 993−999.
-
Li, Y.; Yan, K.; Lee, H.-W.; Lu, Z.; Liu, N.; Cui, Y., Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes., Nat. Energy 2016, 1, 15029.
-
Tan, G.; Xu, R.; Xing, Z.; Yuan, Y.; Lu, J.; Wen, J.; Liu, C.; Ma, L.; Zhan, C.; Liu, Q.; Wu, T.; Jian, Z.; Shahbazian-Yassar, R.; Ren, Y.; Miller, D. J.; Curtiss, L. A.; Ji, X.; Amine, K., Burning Lithium in CS2 for high-performing compact Li2S-graphene nanocapsules for Li-S batteries., Nat. Energy 2017, 2, 17090.
-
Geim, A. K. Graphene: status and prospects. Science 2009, 324(5934), 1530. TEM image in Fig. 4(D) was taken from: Meyer, J., Chuvilin, A., & Kaiser, U. Electron microscopic studies with graphene. Microscopy and Microanalysis 2009, 15(S2), 126.