Understanding metal-graphene interactions
September 04, 2018 – A new study examining graphene-metal interactions and was carried out in the Group of Electron Microscopy for Materials Science at Ulm University, Germany and the Nanoscience & Nanotechnology Centre at Nottingham University, UK. The study uses carbon nanotubes as miniature test tubes to systematically examine the interaction of transition metals with the side walls at atomic resolution.
Metal-graphene interactions studied at atomic resolution is a current aim in high resolution TEM [1], and combined with confinement of metal atoms inside carbon nanotubes provides a powerful strategy for studying structural and chemical properties in detail.[2] The ever-changing nature of the metal nanoclusters during the chemical reactions creates a significant obstacle to the rational design of new nanocatalysts. The only way to assess the relationship between the atomic structure of the nanocluster and its catalytic activity is imaging the dynamic transformations promoted by the metal nanocluster, ideally at the atomic scale. Transmission electron microscopy (TEM) nowadays offers atomic spatial and high temporal resolution, inaccessible in other microscopy methods. With the help of the in-situ TEM techniques, a new study now considers the influence of the e-beam, which has been largely neglected in previous studies.[3-5] It has a dramatic effect on the dynamics and performance of nanocatalysts, as the rates of the reactions are directly proportional to the dose rate of the e-beam.
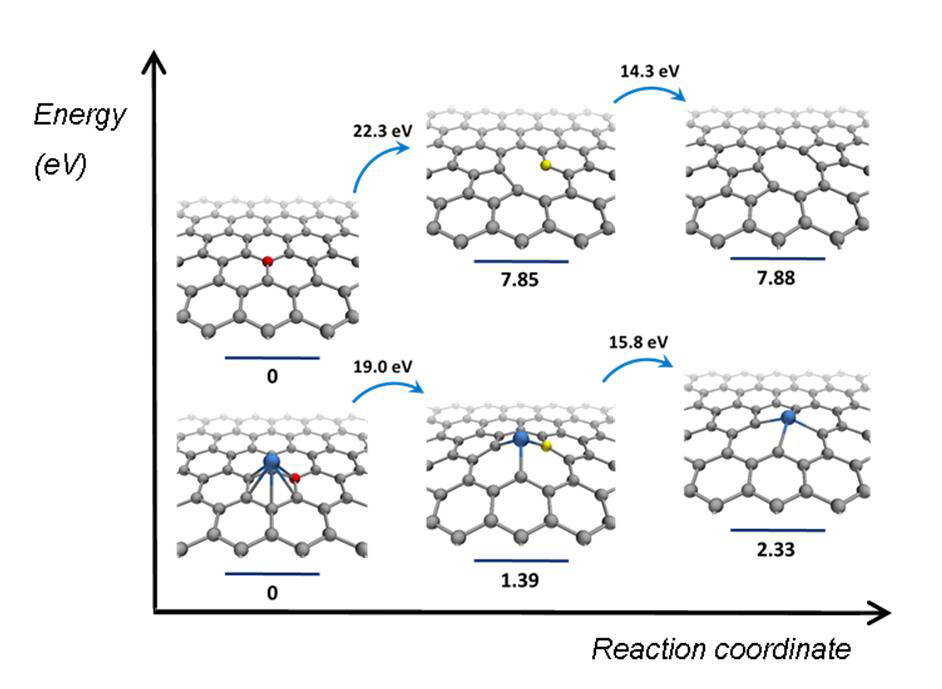
In the new study, TEM images have been combined to form a stop-frame movie bearing information on reaction dynamics and mechanisms at the atomic level.[6] The reactions were driven by isolated events in which direct momentum transfer from a fast incident electron to an atom results in a shift of the atom from its equilibrium position. The exact amount of kinetic energy transferred from the e-beam to the atom has been exactly calculated. The catalytic activity has been studied by observing the restructuring of a surrounding nanotube. For a defect to emerge in the nanotube sidewall, a C–C bond must first be broken, followed by the formation of bond between the metal nanocluster and the dangling bonds of the nanotube (Fig. 1 left). This is depending on the metal nanocluster because: (i) it reduces the energy threshold for carbon atom displacement (Fig. 1 (right) and (ii) it stabilizes the vacancy defect due to the formation of the metal carbon bond (Fig. 1 right). While the latter appears to be more significant, both roles are linked by the Bell-Evans-Polanyi principle—relating the activation energy Ed' to the enthalpy for reactions ∆H' of similar type — which, if applied to the ChemTEM study, (e.g. the lower ∆H' — the lower Ed'; Fig. 1 right), is ultimately defined by the bonding of the metal nanocluster with carbon.
It has been theoretically and experimentally demonstrated previously that the presence of metals can facilitate formation of defects under e-beam irradiation such as mono-vacancy [7, 8] and di-vacancy defects[9, 10] in the graphitic lattice [11, 12]. The authors already conducted ChemTEM studies of individual metals (Ni[13, 14], Re[15]) or metal triads (W-Re-Os[16, 17], Fe-Ru-Os[18]) and found a strong correlation between the observed dynamics of defect formation in carbon structures and the position of the metal in the period or group.[16, 17] Now, in order to analyze this in detail, they conducted a global comparison for the transition metals, revealing fundamental trends across the periodic table.
Using the low-voltage but atomic-resolution TEM equipment to analyze the specially prepared samples, the researchers were able to get a detailed look at the mechanisms of catalysis of nanocatalysts, which play a key role in many industrial processes such as the water-gas shift reaction or methanol synthesis on Cu, and oxygen reduction in fuel cells on Pt/Co, just to name a few. Recent estimates indicate that catalytic chemical reactions account for 30-40% of global gross domestic product (GDP), making the catalytic behavior of nanoclusters at the atomic level an urgent task.
For the study, published Sep. 4 in Nature’s Communications, the researchers analyzed a very large dataset, comprising 14 different transition metals, which allows to identify the systematic changes in the properties for those compounds.
They found why the dynamics type of transition metals and their interaction with carbon nanotubes (catalytic activity) could not be explained so far. The measurements demonstrate no significant correlation between the efficiency of interaction with carbon and the metal’s position in the periodic table. This suggests a complex interplay of the element-specific valence electron configuration, electronegativity and the size of the atom—as discussed recently in comparative theoretical studies of transition metals on carbon,[6-9]. In the new experiments with spatial and temporal resolution, which could not previously be achieved with any other method, the scientists were now able to visualize changes in the atomic structure of the metal nanocluster simultaneously with the carbon structure growth. The momentum transferred by the incident electrons directly to the atoms could purposefully influence the changes.
In general, most of the 14 transition metal nanoclusters which were examined show more dynamic when they begin bonding to carbon. For example, the W and Ni nanoclusters continually and rapidly change as the new carbon structures grow from their surface (Fig. 2, Supplementary Fig. 8, Supplementary Movies 2, 3). This strikingly illustrates the key challenge of nanocatalysis—metal nanoclusters changing their atomic structures during the reaction, that is very similar to the behavior predicted theoretically. [19, 20]
The new observations provide direct evidence of dynamic single-atom catalysis, which was also predicted theoretically.[21] For example, in the arresting behavior of the Pt nanocluster in process 3, the nanocluster acts as a viscous liquid dispersing to individual atoms (Fig. 2, 118 s, marked with the green arrow) and then re-clustering over time (Fig. 2, 238 s). Such individual atoms were expected to coexist with nanocatalysts and play an important role owing to their high surface energy[22].
By analyzing the TEM series (Fig. 2), it was found that the activity of metal nanoclusters towards defect formation in NTs is indeed related to the bonding energy between the nanocluster and carbon, and a volcano-shaped plot was obtained (Fig. 3) — a graphical representation of the Sabatier principle that defines an effective catalytic surface to be neither too reactive nor too inert[23], so that it both binds effectively to the reactants and readily releases the products of the reaction.
The Sabatier principle has become firmly established in the foundations of heterogeneous catalysis[24], guiding the discovery of new catalysts,[25] including those for carbon nanostructure growth.[26] However, because of the highly dynamic surface of metal nanoclusters, there was until now no certainty that this principle would apply also for nanocatalysis. The scientists could now prove that for the 14 transition metals this is indeed the case (Fig. 2), thus verifying that series 1 (Fig. 2) is connected to the relative nanocluster-carbon bonding energy, and that still for nanocatalysts with their tiny size (20–60 atoms) the reaction is closer to the heterogeneous rather than homogeneous catalysts.
The filling of d-orbitals along a period of transition elements results in a sharp decrease in the contribution of the d-orbitals to metallic bonding for Ni, Pd and Pt as compared to other metals.[27] The very high occupancy of the d-orbitals in group 10 allows only transient interactions with surrounding carbon and high diffusivity of carbon atoms through the metal nanocluster, such that no well-defined interface can exist between these metal nanoclusters and carbon structures under demonstrated ChemTEM conditions, thus explaining why the group 10 metals Ni, Pd and Pt appear to disobey the Sabatier principle.
Recent advancements in TEM together with the use of nano test tubes offer a radically new way to study and compare chemical processes at the atomic level.28 “In past studies, they had less information on atomic reactions promoted by nanocatalysts. With filming in the transition electron microscope, we can determine for the first time see the atomic changes of the nanocatalyst and its environment with atomic accuracy. For over six centuries scientists have been using laboratory glassware (test tubes, retorts, flasks, beakers etc.) to discover and investigate the properties of chemical elements and the mechanisms of chemical reactions. These traditional methods work well for reactions where the key parameters stay the same, but in nanocatalysis owing to the largely unpredictable and dynamic behavior of the active center, new approaches are required.” say the study’s senior authors, Ute Kaiser, professor of electron microscopy at Ulm University and Andrey Khlobystov, professor of chemistry at Nottingham University.
ChemTEM methodology applied to transition metals inside carbon nano test tubes provides atomic-level nanocluster dynamics analysis enabled to compare, categorize and order the group of transition metals with respect to their relative catalytic activity and their bonding with carbon. The use of a precisely shaped e-beam to supply the energy to drive chemical reactions and capture their progress in real time coupled with a detailed understanding of the mechanisms [29-31] enables the determination of fundamental thermodynamic parameters[12, 31] and reveals mechanisms of chemical reactions.[15, 32, 33].
“The new study covers the whole block of early and middle transition metals consisting of three periods and five groups, which, for the first time, enabled a detailed analysis of global trends within the family of transition elements.” Says Kecheng Cao from Ulm University, first author of the study. “It reveals that the metal nanoclusters become more dynamic once they are engaged in reactions with carbon—one very unexpected outcomes of this analysis, having significant implications for understanding the atomistic workings of catalytic cycles at the nanoscale. Equally important are our findings that the tiny clusters of metals obey the Sabatier principle as heterogeneous catalysts (with three important exceptions), despite the absence of a well-defined surface, and that different metals exhibit drastically different tendencies towards defect formation in the carbonic lattice of the nanotube.”
This knowledge provides a new guide for the development of better catalysts involving C–C bond dissociation that could be rationally designed and tested on the basis of ChemTEM atomic-scale experiments. Importantly, the strategy of utilizing a confined environment such as a nano test tube to stabilize metal nanoclusters could be utilized in the development of more durable and reusable nanocatalysts. The filming of the highly dynamic nature of metal nanoclusters during reactions is a promising approach to improve the performance of nanocatalysts in the future.[34]
Resource: Cao, K., Zoberbier, T., Biskupek, J., Botos, A., McSweeney, R. L., Kurtoglu, A., Stopiello, C. T., Markevich, A. V., Besley, E., Chamberlain, T. W., Kaiser, U., & Khlobystov, A. N. (2018). Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts. Nature communications, 9: 3382, doi: 10.1038/s41467-018-05831-z, [PDF], see also the supporting information.
Gan, Y., Sun, L., & Banhart, F. (2008). One‐and Two‐Dimensional Diffusion of Metal Atoms in Graphene. Small, 4(5), 587-591.
Chuvilin, A., Khlobystov, A. N., Obergfell, D., Haluska, M., Yang, S., Roth, S., & Kaiser, U. (2010). Observations of Chemical Reactions at the Atomic Scale: Dynamics of Metal‐Mediated Fullerene Coalescence and Nanotube Rupture. Angewandte Chemie International Edition, 49(1), 193-196.
Hansen, P. L., Wagner, J. B., Helveg, S., Rostrup-Nielsen, J. R., Clausen, B. S., & Topsøe, H. (2002). Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science, 295(5562), 2053-2055.
Hofmann, S., Sharma, R., Ducati, C., Du, G., Mattevi, C., Cepek, C., Cantoro, M., Pisana, S., Parvez, A., Cervantes-Sodi, F., Ferrari, A. C., Dunin-Borkowski, R., Lizzit, S., Peaccia, L., Goldoni, A., & Robertson, J. (2007). In situ observations of catalyst dynamics during surface-bound carbon nanotube nucleation. Nano letters, 7(3), 602-608.
Vendelbo, S. B., Elkjær, C. F., Falsig, H., Puspitasari, I., Dona, P., Mele, L., Morana, B., Nlissen, B. J., van Rijn, R., Creemer, J. F., Kooyman, P. J., & Helveg, S. (2014). Visualization of oscillatory behavior of Pt nanoparticles catalyzing CO oxidation. Nature materials, 13(9), 884.
Skowron, S. T., Chamberlain, T. W., Biskupek, J., Kaiser, U., Besley, E., & Khlobystov, A. N. (2017). Chemical reactions of molecules promoted and simultaneously imaged by the electron beam in transmission electron microscopy. Accounts of Chemical Research, 50(8), 1797-1807.
Zhuang, H. L., Zheng, G. P., & Soh, A. K. (2008). Interactions between transition metals and defective carbon nanotubes. Computational Materials Science, 43(4), 823-828.
Inntam, C., & Limtrakul, J. (2010). Adsorption of M species and M2 dimers (M= Cu, Ag, and Au) on the pristine and defective single-walled carbon nanotubes: a density functional theory study. The Journal of Physical Chemistry C, 114(49), 21327-21337.
Krasheninnikov, A. V., Lehtinen, P. O., Foster, A. S., Pyykkö, P., & Nieminen, R. M. (2009). Embedding transition-metal atoms in graphene: structure, bonding, and magnetism. Physical Review Letters, 102(12), 126807.
Boukhvalov, D. W., & Katsnelson, M. I. (2009). Destruction of graphene by metal adatoms. Applied Physics Letters, 95(2), 023109.
Zan, R., Bangert, U., Ramasse, Q., & Novoselov, K. S. (2011). Metal−graphene interaction studied via atomic resolution scanning transmission electron microscopy. Nano letters, 11(3), 1087-1092, and Ramasse, Q. M., Zan, R., Bangert, U., Boukhvalov, D. W., Son, Y. W., & Novoselov, K. S. (2012). Direct experimental evidence of metal-mediated etching of suspended graphene. ACS nano, 6(5), 4063-4071.
Rodríguez-Manzo, J. A., Cretu, O., & Banhart, F. (2010). Trapping of metal atoms in vacancies of carbon nanotubes and graphene. ACS nano, 4(6), 3422-3428.
Lebedeva, I. V., Chamberlain, T. W., Popov, A. M., Knizhnik, A. A., Zoberbier, T., Biskupek, J., Kaiser, U., & Khlobystov, A. N. (2014). The atomistic mechanism of carbon nanotube cutting catalyzed by nickel under an electron beam. Nanoscale, 6(24), 14877-14890.
Sinitsa, A. S., Chamberlain, T. W., Zoberbier, T., Lebedeva, I. V., Popov, A. M., Knizhnik, A. A., McSweeney, R. L., Biskupek, J., Kaiser, U., & Khlobystov, A. N. (2017). Formation of nickel clusters wrapped in carbon cages: Toward new endohedral metallofullerene synthesis. Nano letters, 17(2), 1082-1089.
Chamberlain, T. W., Meyer, J. C., Biskupek, J., Leschner, J., Santana, A., Besley, N. A., Bichoutskaia, E., Kaiser, U., & Khlobystov, A. N. (2011). Reactions of the inner surface of carbon nanotubes and nanoprotrusion processes imaged at the atomic scale. Nature chemistry, 3(9), 732.
Zoberbier, T., Chamberlain, T. W., Biskupek, J., Kuganathan, N., Eyhusen, S., Bichoutskaia, E., Kaiser, U., & Khlobystov, A. N. (2012). Interactions and reactions of transition metal clusters with the interior of single-walled carbon nanotubes imaged at the atomic scale. Journal of the American Chemical Society, 134(6), 3073-3079.
Zoberbier, T., Chamberlain, T. W., Biskupek, J., Suyetin, M., Majouga, A. G., Besley, E., Kaiser, U., & Khlobystov, A. N. (2016). Investigation of the interactions and bonding between carbon and Group VIII metals at the atomic scale. Small, 12(12), 1649-1657.
Yamamoto, A. (1986). Organotransition metal chemistry: fundamental concepts and applications. (Wiley-Interscience: New York), pp. 480.
Ohta, Y., Okamoto, Y., Irle, S., & Morokuma, K. (2008). Rapid growth of a single-walled carbon nanotube on an iron cluster: density-functional tight-binding molecular dynamics simulations. ACS Nano, 2(7), 1437-1444.
Khalilov, U., Bogaerts, A., & Neyts, E. C. (2015). Atomic scale simulation of carbon nanotube nucleation from hydrocarbon precursors. Nature communications, 6, 10306.
Liu, J. C., Wang, Y. G., & Li, J. (2017). Toward rational design of oxide-supported single-atom catalysts: atomic dispersion of gold on ceria. Journal of the American Chemical Society, 139(17), 6190-6199.
Yang, X. F., Wang, A., Qiao, B., Li, J., Liu, J., & Zhang, T. (2013). Single-atom catalysts: a new frontier in heterogeneous catalysis. Accounts of Chemical Research, 46(8), 1740-1748.
Sabatier, P. (1911). Hydrogenation and dehydrogenation by catalysis. Reports of the German Chemical Society, 44(3), 1984-2001.
Nørskov, J. K., Bligaard, T., Hvolbæk, B., Abild-Pedersen, F., Chorkendorff, I., & Christensen, C. H. (2008). The nature of the active site in heterogeneous metal catalysis. Chemical Society Reviews, 37(10), 2163-2171.
Studt, F., Abild-Pedersen, F., Bligaard, T., Sørensen, R. Z., Christensen, C. H., & Nørskov, J. K. (2008). Identification of non-precious metal alloy catalysts for selective hydrogenation of acetylene. Science, 320(5881), 1320-1322.
Robertson, J. (2012). Heterogeneous catalysis model of growth mechanisms of carbon nanotubes, graphene and silicon nanowires. Journal of Materials Chemistry, 22(37), 19858-19862.
Turchanin, M. A., & Agraval, P. G. (2008). Cohesive energy, properties, and formation energy of transition metal alloys. Powder Metallurgy and Metal Ceramics, 47(1-2), 26-39.
Haider, M., Uhlemann, S., Schwan, E., Rose, H., Kabius, B., & Urban, K. (1998). Electron microscopy image enhanced. Nature, 392(6678), 768.
Koshino, M., Niimi, Y., Nakamura, E., Kataura, H., Okazaki, T., Suenaga, K., & Iijima, S. (2010). Analysis of the reactivity and selectivity of fullerene dimerization reactions at the atomic level. Nature chemistry, 2(2), 117.
Barry, N. P., Pitto-Barry, A., Sanchez, A. M., Dove, A. P., Procter, R. J., Soldevila-Barreda, J. J., Kirby, N., Hands-Portsman, I., Smith, C. J., O’Reilly, R. K., Beanland, R., & Sadler, P. J. (2014). Fabrication of crystals from single metal atoms. Nature Communications, 5, 3851.
Gan, Y., Sun, L., & Banhart, F. (2008). One‐and two‐dimensional diffusion of metal atoms in graphene. Small, 4(5), 587-591.
Levy R. B. (1977). Properties of carbides, nitrides, and borides: implications for catalysis. In: Advanced Materials in Catalysis (eds Burton, J. J. Garten, R. L.) (Academic Press, New York), p. 101.
Chuvilin, A., Kaiser, U., Bichoutskaia, E., Besley, N. A., & Khlobystov, A. N. (2010). Direct transformation of graphene to fullerene. Nature chemistry, 2(6), 450.
Cao, B., Starace, A. K., Judd, O. H., & Jarrold, M. F. (2009). Melting dramatically enhances the reactivity of aluminum nanoclusters. Journal of the American Chemical Society, 131(7), 2446-2447.