Observation of lithiated graphene membranes
December 02, 2018 – Since 2004 it has been possible to extract individual carbon layers (graphene) from graphite [1]. Due to its uniform hexagonal atomic lattice and its small thickness, this nanomaterial is particularly well suited as a substrate in transmission electron microscopy [2,3]. In an electrochemical cell of only two graphene sheets [4,5], a much larger lithium mobility than in graphite could be observed for the first time [6] and has now been studied with deep sub-Å resolution by in-situ low-voltage TEM. Using both, spherical (Cs) and chromatic (Cc) aberration correction [7], the observation extends the spectrum of in situ TEM studies on structures made of light elements encapsulated in quasi one-dimensional [8] or two-dimensional [9] materials.
The study of the charging of a battery at atomic resolution is an advance that promises the ability to inspire the design of future carbon-based energy storage systems. The lithium battery whose development began in the 1960s, are characterized by a higher energy density and specific energy, high cell voltage and very long shelf life due to low self-discharge and a wide temperature range for storage and operation. In what the scientists were disagreeing about until now is in what form the lithium is stored and what form the process of intercalation takes. That's because at the atomic level, such a dynamic process is very difficult to observe „in situ“ - i.e. „live“.
Now scientists at the SALVE Microscopy Center have imaged these processes with the graphene cell as a two-dimensional substrate, which is completely impermeable to lithium, and first discovered the phase and dynamics at the root. The new research, led by the group of Jurgen H. Smet from the Max-Planck-Institute for Solid State Research in cooperation with the group of Ute A. Kaiser, head of the SALVE microscopy center from the Central Facility of Electron Microscopy, University of Ulm, was published last week in the journal Nature.
Increasing efforts have been devoted towards imaging lithiation with in situ TEM [10-13], however with only a few studies about the lithiation of pure graphene sheets [14,15]. The research work described further below, has now studied the process in detail and determined the exact structure of an Li bilayer between 2 graphene sheets.
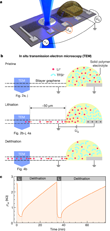
The miniature battery (see schematic including side-view in Figure 1a,b), is supported by Si3N4-covered Si substrate. The Si3N4 forms a 40×40 µm2 large membrane at the center of the Si-chip. The bilayer graphene flake is exfoliated from natural graphite and etched into a Hall bar shape. The end of the graphene flake is connected to a counter electrode via a Li-ion conducting solid polymer electrolyte. Applying a constant voltage UG = 5 V (UG = 0 V) to the counter electrode, lithiates (delithiates) the bilayer graphene. A region uncovered from the electrolyte is partially suspended over a hole formed in the Si3N4 membrane to allow in situ studies in the SALVE (Sub-Ångström Low-Voltage Electron microscopy) microscope [7].
To monitor the lithiation and delithiation also electrically, the scientists contacted the graphene stripe at different distances from the Li-source and monitored the sample resistivity ρ inside the transmission electron microscope. Fig. 1c shows the observed reversible changes of ρxx during subsequent lithiation/delithiation cycles. So as not to damage the graphene cell, an electron acceleration voltage of 80 kV was used, just below the threshold for knock-on damage of C atoms in graphene [16].
Kaiser and her team at Ulm University are already using the graphene cell — fed with sensitive materials that would otherwise be unstable — to observe the structure, vibrational and electrical properties of sensitive materials such as doping or defects in 2D materials [4,5] as well as the dynamical processes such as crystallization on the atomic level [32]. Another advantage of using graphene is that the contrast of the hexagonal lattice can be easily eliminated from the TEM image, making individual molecules and very light elements visible with outstanding contrast and resolution. Also, the extremely high pressure between two graphene sheets [32] leads to confinement of the included material and the formation of phases, which can normally only be observed under very low temperature or high external pressure. Such an effect must be considered for the intercalation in general, making the graphene cell in the TEM ideally suited for battery research.
"Right now, we have the experimental instruments and the theoretical model simulations available — the HRTEM in combination with graphene and Density Functional Theory (DFT) calculations — that a detailed study of the intercalation on the atomic scale becomes possible," Kaiser said. " „The results of this research project are of fundamental importance for battery research, as they provide insights into the course of elementary processes in electrochemical energy storage." Kaiser and Smet agree.
Of great value for the observation in the in-situ TEM is that it provides information on the elemental composition by acquiring EELS data, see Fig. 2j. During lithiation the Li K-edge at 55 eV is observed as in Fig. 2d. On the basis of the absence of other signals in the explored energy range of 0–800 eV—namely, Si (L2,3-edge at 99 eV), S (L2,3-edge at 165 eV), N (K-edge at 400 eV), O (K-edge at 532 eV and F (K-edge at 685 eV) those elements can be discarded as principal constituents of the new crystalline phase. Likewise, Ti and Pt (electrode material) as the would be indicated by higher contrast for Pt in Fig. 2, which was not observed, and the absence of the distinct Ti L2,3-edge at 456 eV in the EELS data for Ti. The analysis also showed a Li plasmon mode [19,20] near 9 eV (Fig. 3a). Though the shape of the Li-K edge [17,18] resembles that of Li2O/LiOH, stoichiometric Li2O/LiOH could be excluded, since we would then expect both a more pronounced O-K edge and an imaging contrast comparable in strength to the encapsulating graphene [21]. The presence of trace oxygen, also suggested by the occasional observation of a weak shoulder near 30 eV (Fig. 3a), previously attributed to oxidized lithium [19,20] can, however, not be ruled out. The elements H and C could not be excluded by the scientists, but the low energy onset of the Li K-edge supports this assertion that the phase formed during lithiation consists of pure Li [17,18].
The Li phase is highly crystalline and stable between perfectly intact graphene planes. Good agreement with the experiment is attained when calculating the Li-K edge shape of graphene-encapsulated Li multilayers (Fig. 3b). Yet, the extracted in-plane lattice constant of 3.1 Å matches that [22] of close-packed Li. This phase is constituting a super dense ordering, which is normally only stable at very low temperatures and/or extreme pressures [23] and was a big surprise to the scientists. In fact, the density of Li is significantly higher than the LiC6 intercalation, that has been previously proposed for the intercalation in graphite.
A back-and-forth controlled exchange
In the new study, the scientists find that regions of different thickness can be identified within a single grain (Fig. 4). When imaging an extremely thin slab of weakly scattering elements in a microscope with enough resolving power, the imaging contrast increases with increasing specimen thickness. This is quantified in Fig. 4e, f, revealing that thinner parts of the close-packed Li phase tend to be located closer to its perimeter. At the leading edge, single atoms can be identified (Fig. 5). From the image time series, a lateral growth rate of the order of 1 Å s−1 can be extracted. During delithiation, the close-packed Li phase disassembles and gradually disappears (Fig. 6b). Eventually, the pure bilayer graphene lattice remains behind.
The degree of reversibility of the process is limited by the number of defects in the graphene lattice and their irreversible formation during prolonged imaging (electron irradiation).
During lithiation, the close-packed Li phase grows laterally between the graphene sheets (Fig. 6 and Video 1). Fig. 6a displays a series of digital dark-field versions of the original images, where the three grains of varying in-plane orientation have each been colored differently. Whereas the grain boundary between the central grain (green) and the lower right grain (blue) is rather sharp and stable, the boundary with the left grain (red) appears fuzzier and more mobile as their orientations nearly match.
Video 1. TEM image sequence TEM image sequence showing the propagation front of a Li crystal forming inside bilayer graphene during lithiation.
Kaiser and her colleagues found that the observed crystalline phase of Li proves stable only between intact graphene planes. When the incident electron energy exceeds the threshold for displacement damage of Li (about 20 keV), conditions are such that Li readily ‘boils’ under the electron beam [17,18,20], the close-packed phase is volatile when imaged near bilayer edges or in the presence of a high density of defects in the graphene lattice.
Constituents may escape from between graphene sheets via such edges or defects on electron-beam-induced melting of the crystalline phase. The protective encapsulation by two impermeable atomic sheets may thus be regarded a prerequisite for safe probing by TEM of the crystal formation therein, akin to the situation in graphene liquid cells [24].
The superdense Li phase
Although enhanced Li storage than LiC6 has been previously proposed to occur on the outside of graphene planes [25,26], the suggested atomistic configurations were contradictory, and could neither be addressed nor verified by microscopic means. Reports claiming the formation of nanocrystals of close-packed Li during the lithiation of different carbon allotropes are scarce and not fully consistent [27,28]. The phase observed now may have been left unidentified in bulk graphite [29], the scientists say. Because the energy cost for close-packing of Li in the van der Waals gap of bilayer graphene is very similar to that of forming C6LiC6 (Fig. 7), close-packing may be the way the system accommodates a large amount of Li supplied within a short time.
Further insights into the atomistic configuration could be gained by DFT calculations. Theoretician at the Research-center Dresden-Rossendorf calculated the activation energy for Li diffusion in bilayer graphene (Fig. 8). It points towards facile diffusion of Li between graphene sheets by the exchange mechanism, even in the presence of a close-packed Li phase. And although a graphene bilayer can be regarded as the structural unit of thicker graphite, its properties differ in many respects. Two atomic sheets may well spread more easily when isolated from their bulk crystal and thus render new types of intercalate ordering possible. The stabilization of a super dense lithium phase comes as a surprise as its appearance has typically been reserved for extreme conditions [23].
The scientists note that Li–graphite intercalation compounds only occupy a small region of the C–Li binary alloy phase diagram [30]. In the miscibility gap beyond LiC6, alternative configurations may be available for storing larger amounts of lithium in layered carbons [22].
"The range of new phases of materials that form between graphene sheets is remarkably complex and can now be studied in detail. This experimental effort has given us new insight into the functioning of a battery." Kaiser said. "More complex sample preparation, with assimilation of more observations in the TEM, will allow us to continue to work on gaining fundamental understanding and discovering of new materials."
Resource: Kühne, M., Börrnert, F., Fecher, S., Ghorbani-Asl, M., Biskupek, J., Samuelis, D., Krasheninnikov, A. V., Kaiser, U. A. & Smet, J. H. (2018). Reversible superdense ordering of lithium between two graphene sheets. Nature, 564, 234-239, doi: 10.1038/s41586-018-0754-2
K. S. Novoselov, A. K. Geim, S. V. Morozov, D. A. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, A. A. Firsov, Electric field effect in atomically thin carbon films. Science 306, 666-669 (2004).
R. S. Pantelic, J. W. Suk, C. W. Magnuson, J. C. Meyer, P. Wachsmuth, U. Kaiser, R. S. Ruoff, H. Stahlberg, Graphene: Substrate preparation and introduction. Journal of structural biology 174, 234-238 (2011).
R. S. Pantelic, J. C. Meyer, U. Kaiser, H. Stahlberg, The application of graphene as a sample support in transmission electron microscopy. Solid State Communications 152, 1375-1382 (2012).
O. Lehtinen, I. L. Tsai, R. Jalil, R. R. Nair, J. Keinonen, U. Kaiser, I. V. Grigorieva, Non-invasive transmission electron microscopy of vacancy defects in graphene produced by ion irradiation. Nanoscale 6, 6569-6576 (2014).
G. Algara-Siller, S. Kurasch, M. Sedighi, O. Lehtinen, U. Kaiser, The pristine atomic structure of MoS2 monolayer protected from electron radiation damage by graphene. Applied Physics Letters 103, 203107 (2013).
M. Kühne, F. Paolucci, J. Popovic, P. M. Ostrovsky, J. Maier, J. H. Smet, Ultrafast lithium diffusion in bilayer graphene. Nature Nanotechnology 12, 895–900 (2017).
M. Linck, P. Hartel, S. Uhlemann, F. Kahl, H. Müller, J. Zach, M. Haider, M. Niestadt, M. Bischoff, J. Biskupek, Z. Lee, R. Lehnert, F. Börrnert, H. Rose, U. Kaiser, Chromatic aberration correction for atomic resolution TEM imaging from 20 to 80 kV. Phys. Rev. Lett. 117, 076101 (2016).
X. H. Liu, Y. Liu, A. Kushima, S. Zhang, T. Zhu, J. Li, J. Y. Huang, In situ TEM experiments of electrochemical lithiation and delithiation of individual nanostructures. Adv. Energy Mater. 2, 722–741 (2012).
J. Wan, S. D. Lacey, J. Dai, W. Bao, M. S. Fuhrer, L. Hu, Tuning two-dimensional nanomaterials by intercalation: materials, properties and applications. Chem. Soc. Rev. 45, 6742–6765 (2016).
Q. Su, D. Xie, J. Zhang, G. Du, B. Xu, In situ transmission electron microscopy observation of the conversion mechanism of Fe2O3/graphene anode during lithiation-delithiation processes. ACS Nano 7, 9115–9121 (2013).
L. Luo, J. Wu, J. Luo, J. Huang, V. P. Dravid, Dynamics of electrochemical lithiation/delithiation of graphene-encapsulated silicon nanoparticles studied by in-situ TEM. Sci. Rep. 4, 3863 (2014).
X.-Y. Shan, G. Zhou, L.-C. Yin, W.-J. Yu, F. Li, H.-M. Cheng, Visualizing the roles of graphene for excellent lithium storage. J. Mater. Chem. A 2, 17808–17814 (2014).
Y. Li, K. Yan, H.-W. Lee, Z. Lu, N. Liu, Y. Cui, Growth of conformal graphene cages on micrometre-sized silicon particles as stable battery anodes. Nat. Energy 1, 15029 (2016).
X. H. Liu, J. W. Wang, Y. Liu, H. Zheng, A. Kushima, S. Huang, T. Zhu, S. X. Mao, J. Li, S. Zhang, W. Lu, J. M. Tour, J. Y. Huang, In situ transmission electron microscopy of electrochemical lithiation, delithiation and deformation of individual graphene nanoribbons. Carbon 50, 3836–3844 (2012).
X. Wang, Q. Weng, X. Liu, X. Wang, D.-M. Tang, W. Tian, C. Zhang, W. Yi, D. Liu, Y. Bando, D. Golberg, Atomistic origins of high rate capability and capacity of N-doped graphene for lithium storage. Nano Lett. 14, 1164–1171 (2014).
J. C. Meyer, F. Eder, S. Kurasch, V. Skakalova, J. Kotakoski, H. J. Park, S. Roth, A. Chuvilin, S. Eyhusen, G. Benner, A. V. Krasheninnikov, U. Kaiser, Accurate measurement of electron beam induced displacement cross sections for single-layer graphene. Phys. Rev. Lett. 108, 196102 (2012).
V. Mauchamp, F. Boucher, G. Ouvrand, P. Moreau, Ab initio simulation of the electron energy-loss near-edge structures at the Li K edge in Li, Li2O, and LiMn2O4. Phys. Rev. B 74, 115106 (2006).
F. Wang, J. Graetz, M. S. Moreno, C. Ma, L. Wu, V. Volkov, Y. Zhu, Chemical distribution and bonding of lithium in intercalated graphite: Identification with optimized electron energy loss spectroscopy. ACS Nano 5, 1190–1197 (2011).
D.-R. Lru, D. B. Williams, The electron-energy-loss spectrum of lithium metal. Phil. Mag. B 53, L123–L128 (1986).
A. Hightower, C. C. Ahn, B. Fultz, P. Rez, Electron energy-loss spectrometry on lithiated graphite. Appl. Phys. Lett. 77, 238–240 (2000).
X. H. Liu, J. W. Wang, Y. Liu, H. Zheng, A. Kushima, S. Huang, T. Zhu, S. X. Mao, J. Li, S. Zhang, W. Lu, J. T. Tour, J. Y. Huang, In situ transmission electron microscopy of electrochemical lithiation, delithiation and deformation of individual graphene nanoribbons. Carbon 50, 3836–3844 (2012).
R. W. G. Wyckof, Crystal structures. Wiley & Sons Vol. 1, 2nd (1963).
G. J. M. Ackland, M. Dunuwille, M. Martinez-Canales, I. Loa, R. Zhang, S. Sinogeikin, W. Cai, S. Deemyad, Quantum and isotope effects in lithium metal. Science 356, 1254–1259 (2017).
J. M. Yuk, J. Park, P. Ercius, K. Kim, D. J. Hellebusch, M. F. Crommie, J. Y. Lee, A. Zettl, A. P. Alivisatos. High-resolution EM of colloidal nanocrystal growth using graphene liquid cells. Science 336, 61–64 (2012).
K. Sato, M. Noguchi, A. Demachi, N. Oki, M. Endo, A mechanism of lithium storage in disordered carbons. Science 264, 556–558 (1994).
M. Deschamps, R. Yazami, Great reversible capacity of carbon lithium electrode in solid polymer electrolyte. J. Power Sources 68, 236–238 (1997).
Q. Wang, H. Li, L. Chen, X. Huang, D. Zhong, E. Wang Investigation of lithium storage in bamboo-like CNTs by HRTEM. J. Electrochem. Soc. 150, A1281–A1286 (2003).
B.-S., Lee, J.-H. Seo, S.-B. Son, S. C. Kim, I.-S. Choi, J.-P. Ahn, K. H. Oh, S.-H. Lee, W.-R. Yu, Face-centered-cubic lithium crystals formed in mesopores of carbon nanofiber electrodes. ACS Nano 7, 5801–5807 (2013).
N. Kambe, M. S. Dresselhaus, G. Dresselhaus, S. Baus, A. R. McGhie, J. E. Fischer, Intercalate ordering in first stage graphite-lithium. Mater. Sci. Eng. 40, 1–4 (1979).
H. Okamoto, Binary alloy phase diagrams. ASM International Vol. 1, (eds T. B. Massalski, H. Okamoto, P. R. Subramanian, L. Kacprzak), p. 857 (1990).
D. Lin, Y. Liu, Z. Liang, H.-W. Lee, J. Sun, H. Wang, K. Yan, J. Xie, Y. Cui, Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nature Nanotechnology 11, 626–632 (2016)
Lehnert, T., Kinyanjui, M. K., Ladenburger, A., Rommel, D., Woerle, K., Boerrnert, F., Leopold, K. & Kaiser, U. (2017). In Situ Crystallization of the Insoluble Anhydrite AII Phase in Graphene Pockets. ACS nano, 11(8), 7967-7973.