Chemical graphene synthesis understood
February 26, 2018 – Clean graphene with lattice defects down to 0.02% has been for the first time reproducibly exfoliated by wet chemistry from natural graphite. A team of German experimental and theoretical scientists could achieve the breakthrough after they had understood the main parameters of graphite intercalation and reduction using computer simulations, Sub-Å Low-Voltage Electron (SALVE) microscopy and Raman spectroscopy.
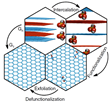
Because of its outstanding electronic and mechanical properties graphene is considered to play a key role in many new advanced technologies. [1][2] High-temperature processes have so far been used to produce high-quality graphene[3], however the production of high-quality graphene with wet-chemical methods was previously impossible. The advantages of a wet-chemical process are its scalability and the ability to simultaneously introduce functional groups, which can give graphene new functions that allow, for example, medical applications[4][5]. But conventional wet-chemical synthesis via graphite intercalation, oxidation, delamination, and subsequent reduction (Figure 1) usually produces high concentrations of defects in the carbon lattice. [6]
Recently it could be demonstrated, that the controlled oxo functionalization of graphene largely prevents the formation of defects in the carbon lattice, resulting in lattice defect concentrations of only 0.3-0.05% . [7][8] This graphene, referred to as oxo G1 (index = number of layers), was changing the game to establish a controlled chemistry of graphene. [9] [10]However, scientists were completely unaware of the processes involved in its synthesis. It was not even known why the oxidation is needed at all and how it influences the chemical reactivity. The intercalation process was sometimes observed to be reversible, [11][12]but other researcher reported the opposite. [8] And the quality of the final product and the yield have been highly variable. [13] Even in one experiment one could observe that the intercalation of graphite was highly inhomogeneous (Figure 2) [11].
Now, scientists at the Universities Erlangen-Nuremberg, Berlin (FU) and Ulm have carried out sophisticated investigation using computer simulations and experiments using high-resolution transmission electron microscopy (HRTEM) and the Raman spectroscopy. The new research that benefited from the wide field of view and high resolution of the SALVE III microscope was published last month in the journal Nature Communications.
Graphite intercalation and graphene preparation
For their systematic study, the team used 3 different types of graphite: natural graphite from Sri Lanka with 70 - 150 μm grain size (NG1), natural graphite with 10 μm grain size (NG2), and turbostratic graphite (TG). NG1 and NG2 have Bernal (AB) or rhombohedral (ABC) stacking, but TG is not regularly stacked.
A clear difference was revealed by Raman spectroscopy of the different graphite types after the intercalation step. For NG1 and NG2, the shift of the G peak to 1630 cm-1 and the almost complete disappearance of the 2D peak was always observed, indicating extremely high doping and thus complete intercalation (Figure 3). [12] In TG the G-peak shift was lower and the D and 2D peaks were still present, demonstrating incomplete intercalation. Furthermore, after flushing with water, the intercalation was irreversible in the case of NG1, partially reversible in the case of NG2 and mostly irreversible in the case of TG, as could be inferred from the Raman spectrum. The spectrum similar to that of graphite oxide with the typical half-widths (Γ ) of the D and G peaks (NG1: ΓD / ΓG = 89) / 81 cm-1; NG2: ΓD / ΓG = 85/70 cm-1; TG: ΓD / ΓG = 97/81 cm-1) [6] was always found in NG1, frequently in NG2, but only in a few cases in TG. In contrast, the spectral features of the original graphite were often reappearing when TG was used (Figure 3).
The preparation of monolayer graphene from the samples is then carried out in ultrasound followed by a chemical reduction. The largest dimensions of graphene flakes (Γ2D <35 cm-1) with diameters of up to 10 μm were found for NG1, whereas the isolation of such flakes for NG2 was only possible sporadically and only with lateral dimensions <3 μm. From TG no graphene flakes could be produced at all in this way.
It is thus possible to obtain monocrystalline graphene without additional purification or heating procedures using NG1. (Figure 4) 34 The HRTEM image shows a few larger holes in an almost intact graphene membrane decorated with amorphous material - probably carbon on both sides of the layer. "Although heteroatoms, such as nitrogen or oxygen, could not be completely excluded, it is a homogeneous atomic lattice with single point defects such as missing C atoms or Stone-Wales defects, which are also observed in graphene made by other methods, such as for example, after decomposition of SiC at 1250 °C", says Prof. Ute Kaiser, who has led the TEM studies at Ulm University.
The effect of charge and friction
In the new study, the scientists carried out extensive ab initio molecular dynamics simulations to find out, why this is working best or even requires AB-stacked NG1 graphite and oxidation. According to the simulations, the initial ability of sulfuric acid to penetrate the graphite lattice is less important than the mobility of sulfuric acid between extended layers of graphite.
The resistance between liquid sulfuric acid and graphite layers is determined by the coefficient of friction defined as the ratio between the frictional force parallel to the carbon layers Fp per unit area A and the velocity jump vslip at the interface [15]. This size can be extracted in an equilibrium simulation using a Green-Kubo relation [16][17].
The leading term in the coefficient of friction is proportional to the square of the ripples of the potential energy surface, which is felt by the sulfuric acid molecules in contact with the graphite layers and thus also influenced by the charge of graphene. [18]
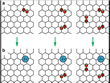
The scientists unraveled several fundamental characteristics of the intercalation process. First they found that under the experimental conditions involving 8 sulfuric acid and one water molecule as well as graphite with a separation distance of 9.12 Å[19], no more than two protons are transferred to a periodic orthorhombic supercell of 60 carbon atoms[20]. This value corresponds very well to the experimentally determined degree of oxidation of intercalated graphite with a positive charge per 24 carbon atoms[13] and can be understood from an analysis of the electronic structure. Additional hydroxyl groups are stable species at higher oxidation states and adhere to the carbon skeleton (Fig. 5).
Second, the friction in AB stacked graphite, where the lattices are offset from each other as in NG1 and NG2 is considerably weaker than in AA stacking, where all the C atoms sit directly on top of each other as in TG (Figure 6b, c). The reason for this is that the AB stack always has a carbon atom below and above a carbon hexagon, which flattens the potential surface. The comparison between the three simulations shows that oxidation (red and blue lines in 6e) and the change of stacking from AA to AB (blue and green lines in 6e) significantly reduce the coefficient of friction.
For the simulations of the oxidized graphite, one H atom per 30 carbon atoms was removed from the sulfuric acid layer. As a result, the waviness in the probability map for the oxidized compared to the neutral system is significantly lower. The origin of this difference is a change in the preferred orientation of the sulfuric acid molecules upon oxidation.
This result is confirmed by an analysis of the xy profiles of the oxygen atom distribution over the carbon atoms. The probability distribution maps po(x,y) shown in Figure 6f were collected by mapping the lateral position of all O atoms along the trajectory on the underlying graphene grid. The graphs for GICAA and ox-GIC (C30+) - AA show the preference for the O atoms of the sulfuric acid species overlying the center of the carbon hexagons.
The probability distribution po(x,y) was converted via ΔA(x,y) = -kBT lnpo(x,y) into a free energy surface from which a line scan (see FIG. 6g) along the line indicated by the arrows in FIG. 6f. From these line scans, oxidation and alteration of the stack sequence from AA to AB significantly reduce the free energy barrier for the sulfuric acid molecules.
"The Chemistry of graphene is remarkably complex, and this experimental and modeling effort has given us some insight into the new structures," Eigler from the Free University Berlin said. "More complex modeling, with assimilation of more observations, will allow us to continue to work on improving the technically highly relevant chemical synthesis of graphene."
Resource: Seiler, S., Halbig, C. E., Grote, F., Rietsch, P., Börrnert, F., Kaiser, U., Meyer, B. & Eigler, S. (2018). Effect of friction on oxidative graphite intercalation and high-quality graphene formation. Nature communications, 9: 836, doi: 10.1038/s41467-018-03211-1, [PDF], see also the supporting information.
Novoselov, K. S., Fal, V. I., Colombo, L., Gellert, P. R., Schwab, M. G., & Kim, K. (2012). A roadmap for graphene. Nature, 490(7419), 192.
Eigler, S., & Hirsch, A. (2014). Chemistry with graphene and graphene oxide—challenges for synthetic chemists. Angewandte Chemie International Edition, 53(30), 7720-7738.
Garcia-Hernandez, M., & Coleman, J. (2016). Materials science of graphene: a flagship perspective. 2D Materials, 3(1), 010401.
Georgakilas, V., Otyepka, M., Bourlinos, A. B., Chandra, V., Kim, N., Kemp, K. C., Hobza, P., Zboril, R., & Kim, K. S. (2012). Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chemical reviews, 112(11), 6156-6214.7.
Mao, H. Y., Laurent, S., Chen, W., Akhavan, O., Imani, M., Ashkarran, A. A., & Mahmoudi, M. (2013). Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chemical reviews, 113(5), 3407-3424.
Eigler, S., Dotzer, C., & Hirsch, A. (2012). Visualization of defect densities in reduced graphene oxide. Carbon, 50(10), 3666-3673.
Eigler, S., Enzelberger‐Heim, M., Grimm, S., Hofmann, P., Kroener, W., Geworski, A., Dotzer, C., Röckert, M., Xiao, J., Papp, C., Lytken, O., Steinrück, H. P., Müller, P., & Hirsch, A. (2013). Wet chemical synthesis of graphene. Advanced Materials, 25(26), 3583-3587.
Eigler, S. (2015). Graphite sulphate–a precursor to graphene. Chemical Communications, 51(15), 3162-3165.
Eigler, S., Grimm, S., Hof, F., & Hirsch, A. (2013). Graphene oxide: a stable carbon framework for functionalization. Journal of Materials Chemistry A, 1(38), 11559-11562.
Eigler, S. (2016). Controlled chemistry approach to the oxo-functionalization of graphene. Chemistry–A European Journal, 22(21), 7012-7027.
Dimiev, A. M., Bachilo, S. M., Saito, R., & Tour, J. M. (2012). Reversible formation of ammonium persulfate/sulfuric acid graphite intercalation compounds and their peculiar Raman spectra. ACS nano, 6(9), 7842-7849.
Dimiev, A. M., Ceriotti, G., Behabtu, N., Zakhidov, D., Pasquali, M., Saito, R., & Tour, J. M. (2013). Direct real-time monitoring of stage transitions in graphite intercalation compounds. ACS nano, 7(3), 2773-2780.
Rüdorff, W., & Hofmann, U. (1938). Über Graphitsalze. Zeitschrift für anorganische und allgemeine Chemie, 238(1), 1-50.
Butz, B., Dolle, C., Halbig, C. E., Spiecker, E., & Eigler, S. (2016). Highly intact and pure oxo-functionalized graphene: synthesis and electron-beam-induced reduction. Angewandte Chemie International Edition, 55(51), 15771-15774.
Tocci, G., Joly, L., & Michaelides, A. (2014). Friction of water on graphene and hexagonal boron nitride from ab initio methods: very different slippage despite very similar interface structures. Nano letters, 14(12), 6872-6877.
Bocquet, L., & Barrat, J. L. (1994). Hydrodynamic boundary conditions, correlation functions, and Kubo relations for confined fluids. Physical Review E, 49(4), 3079.
Bocquet, L., & Barrat, J. L. (2013). On the Green-Kubo relationship for the liquid-solid friction coefficient. The Journal of Chemical Physics, 139(4), 044704.
Bocquet, L., & Charlaix, E. (2010). Nanofluidics, from bulk to interfaces. Chemical Society Reviews, 39(3), 1073-1095.
Dimiev, A. M., & Tour, J. M. (2014). Mechanism of graphene oxide formation. ACS nano, 8(3), 3060-3068.
Yan, J. A., Xian, L., & Chou, M. Y. (2009). Structural and electronic properties of oxidized graphene. Physical Review Letters, 103(8), 086802.