Charge transfer from carbon hosts to encapsulated metal oxides in TEM
May 14, 2025 – Researchers from the University of Nottingham (UK), Ulm University (Germany), and the Universidad de Valencia (Spain) have unveiled a model system in which metal-oxide nanoparticles encapsulated in single-walled carbon nanotubes (SWNTs) exhibit high electrocatalytic activity for water splitting. Remarkably, even though the encapsulated oxides are isolated from the bulk electrolyte, the carbon shell alone shows metal-oxide-like catalytic performance. Spectroelectrochemical analysis and first-principles simulations reveal that charge transfer from the carbon host to the metal oxide reduces the electron density on the carbon surface, thereby enhancing its oxophilicity and accelerating the rate-determining step of the oxygen evolution reaction (OER). These insights provide a new mechanistic framework for designing efficient and sustainable carbon-based electrocatalysts through electronic modulation.
Electrolysis of water involves the oxygen evolution reaction (OER) at the anode and the hydrogen evolution reaction (HER) at the cathode.1,2 A major challenge in this process lies in the limited stability and short lifetimes of conventional OER electrocatalysts, which degrade rapidly under highly oxidizing conditions at the anode.3 State-of-the-art OER electrocatalysts typically rely on noble metals or their oxides—such as platinum (Pt), ruthenium dioxide (RuO2), and iridium dioxide (IrO2)—which are expensive and scarce.4,5 To address this, recent research has focused on minimizing the use of precious metals, improving catalyst longevity, or replacing them entirely with more abundant alternatives. One promising approach involves nanostructured electrocatalysts supported on conductive carbon substrates, which can significantly enhance both stability and activity. This strategy has enabled the use of first-row transition metals such as cobalt (Co), iron (Fe), and nickel (Ni), yielding catalytic activities that rival those of conventional noble-metal-based systems.6–11
A particularly powerful and emerging strategy to enhance the activity and durability of electrocatalysts involves their encapsulation within carbon shells.12–14 A similar approach has also been applied to the development of electrocatalysts coated with monolayers of hexagonal boron nitride (hBN).15 Many researchers focus on increasing the porosity of the carbon support to enhance access to the encapsulated metal centers, which are commonly assumed to serve as the catalytic sites.16 Others aim to modulate the electronic structure of the carbon support itself—an idea first proposed by Deng and colleagues17—based on the hypothesis that electrocatalysis may occur at the carbon shell surrounding the metal centers.11,18 It has been suggested that charge transfer between the encapsulated species and the carbon coating can alter the work function of carbon atoms adjacent to the encapsulated material, as well as those in its vicinity,17,37 similar to the effects observed with heteroatom doping of carbon lattices.19 Computational studies further indicate that the charge transfer effect is maximized with a single atomic layer of carbon and decreases significantly with additional layers.17 Charge transfer also influences the electronic structure and catalytic behavior of metal surfaces. For instance, the remarkable activity of platinum-on-carbon (Pt/C) electrocatalysts has been attributed to electron transfer from Pt d orbitals to the carbon π* orbitals, which lowers the energy barrier for water dissociation at electron-deficient platinum sites.20 Nonetheless, there are also systems in which both the metal and carbon components contribute to catalytic activity.21 Computational models yield conflicting predictions regarding the location of active sites: while Li et al. suggested that iron oxide confined within single-walled carbon nanotubes (SWNTs) serves as the active site for OER,22 Ma’s simulations indicate that oxygen reduction occurs at the carbon surface in carbon–iron composites.23 Recently, Prato and collaborators emphasized the importance of experimental validation to identify active sites in such systems and cautioned against assumptions that prioritize one site over another.24
In the new study, redox-active materials were encapsulated within single-walled carbon nanotubes (SWNTs)25—and previously within multi-walled nanotubes—17,26,27 for use in electrocatalytic processes such as the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER).28 Arc-discharge SWNTs with a narrow diameter range of 1.4–1.5 nm and lengths exceeding 1 μm were selected as catalyst hosts due to their low defect densities and uniform geometry. These features are critical for interpreting bulk electrocatalytic activity mechanistically and for minimizing electrolyte penetration to the inner walls and metal oxide surfaces.
To eliminate interference from metallic residues, SWNT end caps and residual nickel catalyst were removed through sequential treatment in 3.0 M HNO3, thermal oxidation in air, and concentrated HCl washing (Fig. 1a).29,30 Thermogravimetric analysis (TGA) confirmed a post-combustion residue of less than 0.2%, and X-ray photoelectron spectroscopy (XPS) showed no detectable Ni signal, confirming the removal of metal impurities. Encapsulation of the metal oxides was accomplished by subliming metal carbonyls (Mx(CO)y) into the nanotubes under reduced pressure, followed by oxidative treatment in air. These compounds are ideal precursors due to their volatility and small molecular dimensions, which allow for efficient filling of the 1.4–1.5 nm-wide SWNT cavities (Fig. 1b–d).31,32
The catalytic activities of all MOx@SWNT materials exceeded those of pristine SWNTs and aligned with or surpassed prior reports for Co3O4,33 RuO2,14,34,35 and IrO2,14,36 despite the electrocatalysts being confined within the nanotube structure (Fig. 2a). Tafel slope analysis from RDE voltammetry (Fig. 2b) revealed oxygen evolution reaction (OER) slopes of ~100 mV dec–1 for Co3O4@SWNT, IrO2@SWNT, and pristine SWNTs, suggesting a shared rate-determining step (RDS). These values correspond to a mechanism where –OH ion binding constitutes the RDS,37,38 and RuO2@SWNT showed a higher Tafel slope, attributed to concurrent oxidation of RuO2 at the OER onset. Importantly, these slopes are considerably greater than those typically observed for metal oxides (~40–55 mV dec–1),39 indicating that the carbon surface—not the encapsulated metal—likely mediates catalytic activity.
To evaluate stability, Co3O4@SWNT, RuO2@SWNT, and IrO2@SWNT were cycled 2000 times between 0.8 and 1.7 V (vs. RHE) in N2-saturated 0.10 M KOH at 100 mV s–1. Post-cycling voltammetry (Fig. 2c–f) revealed negligible change for Co3O4@SWNT and RuO2@SWNT, whereas IrO2@SWNT showed a reduction in η10. The OER Tafel slopes decreased to 83, 67, and 77 mV dec–1 for Co3O4@SWNT, RuO2@SWNT, and IrO2@SWNT, respectively. This behavior is likely due to partial loss of the SWNT wall or formation of oxygenated surface groups that locally alter electronic density, thereby enhancing –OH binding.19
Pristine SWNTs also showed a notable loss in activity after 2000 cycles (Fig. 2f), likely due to oxidative carbon degradation. Nonetheless, Raman spectroscopy detected fewer than 20 defects per μm in the SWNTs post-cycling, and ICP analysis confirmed no measurable metal ion leaching. To benchmark performance, MOx analogues were loaded on activated carbon (MOx/AC), which initially matched the activity of MOx@SWNTs but degraded significantly upon cycling (Fig. 2c–e). Finally, KSCN—an inhibitor that binds to metal oxide surfaces—was introduced to the electrolyte. It had no effect on MOx@SWNT activity but drastically reduced the activity of MOx/AC, thereby confirming that the carbon surface governs electrocatalysis in the encapsulated systems.40
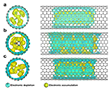
Charge transfer between the encapsulated metal oxides and the carbon host was investigated using first-principles density functional theory (DFT) calculations via the CP2K/QUICKSTEP framework.41,42 The study modeled the confinement of Co3O4, RuO2, and IrO2 fragments within (11,11) SWNTs to quantify electronic density changes upon encapsulation. Charge density difference maps (Fig. 3) illustrate a systematic transfer of electron density from the delocalized π-electron system of the carbon nanotube to the metal oxide units. The charge redistribution predominantly occurred toward the terminal oxygen atoms of the MOx, driven by their high electronegativity.
Notably, the charge transfer was not uniform across the interface; the greatest transfer was observed at oxygen atoms positioned closest to the inner walls of the SWNT (Fig. 3b,c). This spatial variation indicates that both the morphology and electronic structure of the encapsulated species influence the extent and distribution of interfacial charge transfer. These findings confirm that encapsulation within carbon nanostructures significantly modulates the electronic environment of metal oxides and offers a mechanism for enhancing electrocatalytic performance.
In addition, variations in charge-transfer resistance under oxygen evolution reaction (OER) conditions were detected (Fig. 4), and these differences were dependent on the specific identity of the encapsulated MOx@SWNT materials.
Resource:
Townsend, W. J. V., López-Alcalá, D., Bird, M. A. et al. (2025).
The role of carbon catalyst coatings in the electrochemical water splitting reaction.
Nature Communications, 16, 4460.
https://doi.org/10.1038/s41467-025-59740-z
-
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P., & Markovic, N. M. (2017). Energy and fuels from electrochemical interfaces. Nature Materials, 16(1), 57–69.
-
Song, F., Bai, L., Moysiadou, A., Lee, S., Hu, C., Liardet, L., & Hu, X. (2018). Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. Journal of the American Chemical Society, 140(25), 7748–7759.
-
Kötz, R., Lewerenz, H. J., & Stucki, S. (1983). XPS studies of oxygen evolution on Ru and RuO2 anodes. Journal of The Electrochemical Society, 130(4), 825.
-
Shi, Z., Wang, X., Ge, J., Liu, C., & Xing, W. (2020). Fundamental understanding of the acidic oxygen evolution reaction: mechanism study and state-of-the-art catalysts. Nanoscale, 12(25), 13249–13275.
-
Naito, T., Shinagawa, T., Nishimoto, T., & Takanabe, K. (2021). Recent advances in understanding oxygen evolution reaction mechanisms over iridium oxide. Inorganic Chemistry Frontiers, 8(11), 2900–2917.
-
Suen, N. T., Hung, S. F., Quan, Q., Zhang, N., Xu, Y. J., & Chen, H. M. (2017). Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chemical Society Reviews, 46(2), 337–365.
-
Hu, C., Zhang, L., & Gong, J. (2019). Recent progress made in the mechanism comprehension and design of electrocatalysts for alkaline water splitting. Energy & Environmental Science, 12(9), 2620–2645.
-
Exner, K. S. (2019). Design criteria for oxygen evolution electrocatalysts from first principles: introduction of a unifying material-screening approach. ACS Applied Energy Materials, 2(11), 7991–8001.
-
Exner, K. S. (2020). Universality in oxygen evolution electrocatalysis: High‐throughput screening and a priori determination of the rate‐determining reaction step. ChemCatChem, 12(7), 2000–2003.
-
Zhao, Q., Yan, Z., Chen, C., & Chen, J. (2017). Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chemical Reviews, 117(15), 10121–10211.
-
Wang, X., Li, Z., Qu, Y., Yuan, T., Wang, W., Wu, Y., & Li, Y. (2019). Review of metal catalysts for oxygen reduction reaction: from nanoscale engineering to atomic design. Chem, 5(6), 1486–1511.
-
Deng, J., Deng, D., & Bao, X. (2017). Robust catalysis on 2D materials encapsulating metals: concept, application, and perspective. Advanced Materials, 29(43), 1606967.
-
Peng, Y., & Chen, S. (2018). Electrocatalysts based on metal@carbon core@shell nanocomposites: An overview. Green Energy & Environment, 3(4), 335–351.
-
Shen, L., Ying, J., Ozoemena, K. I., Janiak, C., & Yang, X. Y. (2022). Confinement effects in individual carbon encapsulated nonprecious metal‐based electrocatalysts. Advanced Functional Materials, 32(15), 2110851.
-
Velicky, M., et al. (2019). Electron tunneling through boron nitride confirms Marcus–Hush theory predictions for ultramicroelectrodes. ACS Nano, 14(1), 993–1002.
-
Kim, Y., Jeffery, A. A., Min, J., & Jung, N. (2019). Modulating catalytic activity and durability of PtFe alloy catalysts for oxygen reduction reaction through controlled carbon shell formation. Nanomaterials, 9(10), 1491.
-
Deng, D., et al. (2013). Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angewandte Chemie International Edition, 52, 371–375.
-
Kosmala, T., et al. (2021). Operando visualization of the hydrogen evolution reaction with atomic-scale precision at different metal–graphene interfaces. Nature Catalysis, 4, 850–859.
-
Zhao, Z., & Xia, Z. (2016). Stable ultrathin-shell double emulsions for controlled release. ACS Catalysis, 6, 1553–1558.
-
Gupta, G., et al. (2010). Highly stable Pt/ordered graphitic mesoporous carbon electrocatalysts for oxygen reduction. Journal of Physical Chemistry C, 114, 10796–10805.
-
Wen, Z., Liu, J., & Li, J. (2008). Core/Shell Pt/C nanoparticles embedded in mesoporous carbon as a methanol‐tolerant cathode catalyst in direct methanol fuel cells. Advanced Materials, 20, 743–747.
-
Li, Y., Lu, X., Li, Y., & Zhang, X. (2018). Oxygen evolution reaction in nano confined carbon nanotubes. Physica E: Low-dimensional Systems and Nanostructures, 99, 1–5.
-
Ma, D., Jia, S., Zhao, D., Lu, Z., & Yang, Z. (2014). O2 activation on the outer surface of carbon nanotubes modified by encapsulated iron clusters. Applied Surface Science, 300, 91–97.
-
Melchionna, M., Fornasiero, P., & Prato, M. (2020). Into the carbon: A matter of core and shell in advanced electrocatalysis. APL Materials, 8, 020905.
-
Jordan, J. W., et al. (2021). Electrochemistry of redox-active molecules confined within narrow carbon nanotubes. Chemical Society Reviews, 50, 10895–10916.
-
Wang, M., et al. (2021). Endohedral Fe3C decorated multi-walled CNTs as an efficient electrocatalyst for oxygen evolution. Diamond and Related Materials, 118, 108508.
-
Song, L., et al. (2021). Facile synthesis of Co, N enriched carbon nanotube and active site identifications for bifunctional oxygen reduction and evolution catalysis. Energy Storage Materials, 43, 365–374.
-
Cui, T., et al. (2019). Enhanced hydrogen evolution reaction over molybdenum carbide nanoparticles confined inside single-walled carbon nanotubes. Journal of Energy Chemistry, 28, 123–127.
-
Pumera, M., & Iwai, H. (2009). Metallic impurities within residual catalyst metallic nanoparticles are in some cases responsible for "electrocatalytic" effect of carbon nanotubes. Chemistry – An Asian Journal, 4, 554–560.
-
Pumera, M., & Miyahara, Y. (2009). What amount of metallic impurities in carbon nanotubes is small enough not to dominate their redox properties? Nanoscale, 1, 260–265.
-
Botos, A., et al. (2016). Carbon nanotubes as electrically active nanoreactors for multi-step inorganic synthesis: sequential transformations of molecules to nanoclusters and nanoclusters to nanoribbons. Journal of the American Chemical Society, 138, 8175–8183.
-
McSweeney, R. L., et al. (2016). Direct measurement of electron transfer in nanoscale host-guest systems: metallocenes in carbon nanotubes. Chemistry – A European Journal, 22, 13540–13549.
-
Liu, W., Kamiko, M., Yamada, I., & Yagi, S. (2022). Electrochemical deposition of amorphous cobalt oxides for oxygen evolution catalysis. RSC Advances, 12, 8731–8736.
-
Gao, X., et al. (2020). Ru/RuO2 nanoparticle composites with N-doped reduced graphene oxide as electrocatalysts for hydrogen and oxygen evolution. ACS Applied Nano Materials, 3, 12269–12277.
-
Zhang, L., et al. (2022). Efficient oxygen evolution reaction on RuO2 nanoparticles decorated onion-like carbon. Nanotechnology, 33, 135710.
-
Sun, H., et al. (2017). B‐site cation ordered double perovskites as efficient and stable electrocatalysts for oxygen evolution reaction. Chemistry – A European Journal, 23, 5722–5728.
-
Shinagawa, T., Garcia-Esparza, A. T., & Takanabe, K. (2015). Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Scientific Reports, 5, 13801.
-
Cheng, Y., et al. (2023). Oxygen evolution reaction kinetics and mechanisms on pristine carbon nanotubes: Effect of pH. Electrochimica Acta, 438, 141146.
-
Haase, F. T., et al. (2022). Size effects and active state formation of cobalt oxide nanoparticles during the oxygen evolution reaction. Nature Energy, 7, 765–773.
-
Zhao, H., et al. (2023). Fine-tuning surface oxidation states of ruthenium nanoparticles to enhance hydrogen electrode reactions. Journal of Materials Chemistry A, 11, 18262–18271.
-
Hutter, J., Iannuzzi, M., Schiffmann, F., & VandeVondele, J. (2014). cp2k: atomistic simulations of condensed matter systems. WIREs Computational Molecular Science, 4, 15–25.
-
Kühne, T. D., et al. (2020). CP2K: An electronic structure and molecular dynamics software package – Quickstep: Efficient and accurate electronic structure calculations. Journal of Chemical Physics, 152, 194103.